Abstract
Background Thrombopoietin receptor agonists (TPO-RAs) are the recommended second line treatment in most guidelines for immune thrombocytopenia (ITP) allowing patients to avoid immunosuppressive treatments (Provan et al and Neunert et al 2019).
Three different TPO-RAs are licensed for use in ITP. Real world evidence has shown that if patients do not respond to one agent, they may respond to another. This is thought to relate to different signalling pathways, and possibly one TPO-RA creating a stronger signal than another (Olnes et al, 2012; D'Arena et al, 2013). Nonetheless, it is difficult to fully explain responses on switching agents, especially a response to Eltrombopag (ELT) when Romiplostim (ROMI) has failed.
In addition to being a TPO-RA, ELT is a small molecule peptide, which chelates cations, in particular iron and calcium. We have previously shown its ability to shuttle iron out of cells, and the impact this has on liver, cardiac and megakaryocyte cells in vitro (Roth et al, 2012; Vlachodimitropoulou et al, 2016, Liu et al 2022).
We previously reported that ELT has an anti-proliferative effect on stimulated Jurkat and primary T cells, and demonstrated that patients on ELT have a reduced frequency of terminally differentiated effector (TEMRA) CD8 T cells compared to patients on ROMI (Sayed et al, ASH, 2019). We propose that this is related to an intracellular iron chelation effect, which could either explain or be additive to its megakaryocyte stimulating effect in ITP. In this study, we further explored the immunomodulatory effect of ELT on CD8 T cells in ITP.
Methods Primary T cells were stimulated with Dynabeads Human T-Activator CD3/CD28 in the presence of ELT, ROMI, Deferoxamine (DFX) or human recombinant TPO (rhTPO) at increasing doses based on their calculated therapeutic serum levels. DFX and rhTPO act as positive control for iron chelation and TPO-R, respectively. Cell cycle was measured using Propidium Iodide (PI) and analysed using flow cytometry. To explore the in vivo effect of ELT, we measured CD8 TEMRA cells before and after treatment in a cohort of patients with ITP treated with ELT.
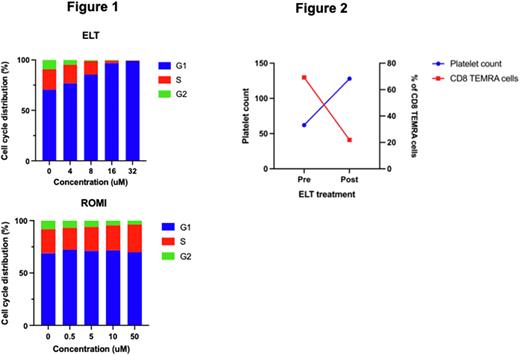
Results Primary T cells treated with ELT resulted in cell cycle arrest to G1 phase. This effect was more pronounced with increasing doses of ELT. DFX exhibited similar effect on primary T cells, however, the induction of G1 phase cell cycle arrest was less potent than ELT. ROMI and rhTPO did not affect cell cycle progression of primary T cells (Figure 1). To confirm whether this cell cycle arrest was due to the iron chelating properties of ELT, we treated the arrested primary T cells with ammonium iron (III) for 24 hours, and found that cell cycle was able to be rescued.
We further investigated the immunomodulatory effect of ELT in patients with ITP by assessing CD8 TEMRA cells (CD45RA+CD62L-) before and after ELT treatment. We found that patients who responded to ELT had a reduction in the frequency of TEMRA cells correlating to their platelet count. An example shown in Figure 2 of a patient who had previously failed ROMI, Mycophenolate Mofetil (MMF) and Rituximab, and subsequently responded to ELT. Prior to ELT, at low platelet count, patient presented with high frequency of CD8 TEMRA cells. Response to ELT resulted in an increased platelet count and a corresponding fall in CD8 TEMRA cells.
Discussion Here we show that ELT suppresses the proliferation of T cells, through cell cycle arrest via its iron chelating properties. We propose that this could have a dual impact in patients with ITP, increasing platelet production as well as dampening down the immune response, hence explaining why switching to ELT could be effective if other TPO-RAs have failed.
Disclosures
Cooper:Sanofi, Principia, Novartis, Griffols, Sobi, Argenyx, UCB, Rigel: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal